
- COBALT ELECTRON CONFIGURATION SPY FULL
- COBALT ELECTRON CONFIGURATION SPY PLUS
- COBALT ELECTRON CONFIGURATION SPY FREE
Exposure to cobalt-60, a powerful gamma ray emitter, may cause cancer. Cobalt should be handled with care because of its toxicity and its risk factor in nuclear confrontation. In small amounts, cobalt is an essential element for humans and many other living organisms, and it is also a central component of vitamin B-12 or cobalamin. Brandt between 17 when he was able to show that cobalt colors glass a rich blue. This solid ferromagnetic silver-white element was known in ancient times for its compounds, but its discovery was credited to G. A bridging ligand donates one electron towards bridging metal atom.Obtained from: arsenic, oxygen, sulfur, cobatineįrequently, cobalt is associated with nickel because both elements have characteristic ingredients of meteoric iron. The electronic compartment (E-bay) was located just aft of the pilots station. including security configurations, before being installed into TSMCs. For example, to find the configuration for the lithium ion (Li), start with neutral lithium (1s2s).
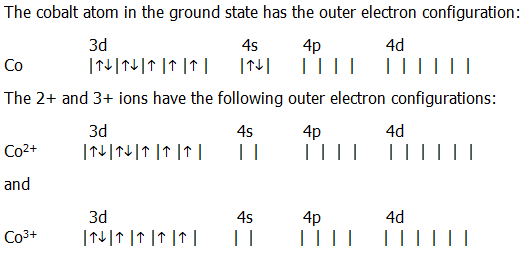
Then, add or remove electrons depending on the ion's charge. Please note that a metal-metal bond contributes one electron to the total electron count of the metal atom. performance of an optimum configuration at speeds of up to Mach 3.5. To find the electron configuration for an ion, first identify the configuration for the neutral atom. The counting of the 18 valence electrons in transition metal complexes may be obtained by following either of the two methods of electron counting, ( i).
COBALT ELECTRON CONFIGURATION SPY PLUS
The 18 Valence Electron (18 VE) Rule or The Inert Gas Rule or The Effective Atomic Number (EAN) Rule: The 18-valence electron (VE) rule states that thermodynamically stable transition metal compounds contain 18 valence electrons comprising of the metal d electrons plus the electrons supplied by the metal bound ligands.
COBALT ELECTRON CONFIGURATION SPY FREE
Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies.
COBALT ELECTRON CONFIGURATION SPY FULL
The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Partial filling of these orbitals thus render these metal centers both electron donor and electron acceptor abilities, thus allowing them to participate in σ-donor/π-acceptor synergic interactions with donor-acceptor ligands like carbonyls, carbenes, arenes, isonitriles and etc. Electron configuration chart of all Elements is mentioned in the table below. port for treaty monitoring, and electronic warfare, is head.

1.electron configuration of K192.electron configuration of Zn303.electron configuration of S164.electron configuration of Ba56paanswer salamat 13. zational structure of modern espionage, intelligence, and security. Unlike the main group organometallic compounds, which use mainly ns and np orbitals in chemical bonding, the transition metal compounds regularly use the ( n−1) d, ns and np orbitals for chemical bonding (Figure 1). Cobalt is a chemical element of the periodic table with chemical symbol Co and atomic number 27 with an atomic weight of 58.9332 u and is classed as a transition metal. electron configuration Cl17electron configuration Co27electron configuration Ge32please po Ty 12. Many of these transition metal organometallic compounds are primarily of interest from the prospectives of chemical catalysis. Study the Cobalt Electron Configuration and get to know this chemical element from a closure perspective. The transition metal organometallic compounds exhibit diverse structural variations that manifest in different chemical properties.

Primary XPS region: Co2p Overlapping regions: Co LMM, Ba3d Binding energies of common chemical states: Chemical state Binding energy Co2p 3/2 Co metal: 778. Leviathan is an espionage actor targeting organizations and high-value targets in. Have an insight about the stability of the transition metal complexes with respect to their total valence electron count. Cobalt X-ray photoelectron spectra, cobalt electron configuration, and other elemental information.
In this lecture you will learn the following


 0 kommentar(er)
0 kommentar(er)
